From shaking a bottle of salad dressing to mixing a can of paint, we interact with emulsions—defined as a blend of two liquids that typically don’t mix, such as oil and water—daily.
For a vast range of foods and other technologies, scientists have devised emulsifying agents which help stabilize mixtures. By incorporating small granular particles to certain foods, it can help prevent spoilage and extend shelf life, important for safeguarding our food supply. When added to chemical mixtures, emulsifying agents can reduce viscosity, making liquids such as petroleum easier to pump and transport through pipelines, potentially leading to energy savings.
Researchers are continually investigating new emulsifiers to improve the control of liquid-liquid mixtures. Recently, Joseph Paulsen, a physics professor in the College of Arts and Sciences, collaborated with scientists from the University of Massachusetts Amherst and Tufts University to make a surprising discovery.

They found that when magnetized particles are added to a simple oil-and-water “salad dressing,” the mixture consistently separates into patterns resembling the elegant curves of a Grecian urn immediately after being shaken. The team’s results, published in Nature Physics, uncover a novel method of using magnetic particles to control liquid-liquid mixtures.
The study, led by UMass Amherst, began when UMass graduate student Anthony Raykh was experimenting in the lab. He added magnetized nickel particles to a batch of “salad dressing” instead of spices, which are normally what allow the oil and water in dressing to remain mixed. He chose magnetized particles because fluids containing them can be engineered to exhibit unique and useful properties. After shaking his mixture, Raykh was astonished to see it consistently form a pristine urn shape. Regardless of how many times or how vigorously he shook the mixture, the urn shape always reappeared.
To help explain this shocking phenomenon, the UMass team invited in Paulsen from Syracuse, along with colleagues from Tufts, to conduct theoretical analysis and simulations. Paulsen, whose research focuses on soft condensed matter, explores the ways in which materials like liquids and soft solids bend, deform and mix—research which lent itself well to this study.

Typically, particles added to an oil-and-water mixture, such as spices, decrease the tension at the interface between the two liquids, allowing them to mix. But in a twist, the team found that particles that are magnetized strongly enough actually increase the interfacial tension, bending the boundary between oil and water into a graceful curve.
“We turned the nature of particle-decorated interfaces on its head,” says Paulsen. “Now, you can have an emulsion droplet that you can imagine controlling in a variety of ways with a magnetic field, but the droplet will nevertheless coalesce with other droplets — something that particle-coated droplets typically resist.”
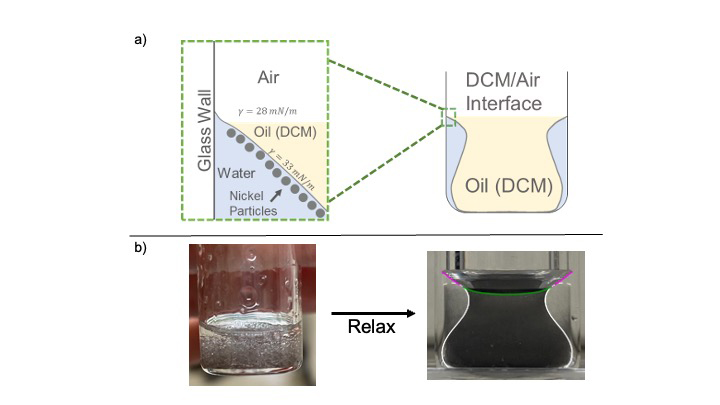
Their research on magnetic particles uncovered two surprising effects. First, the particles, being small magnets, form large networks with many holes due to magnetic interactions. These holes help droplets coated with the particles merge quickly into single oil and water portions. Second, the strong attraction between the magnetic particles increases the surface tension at the interface, further promoting droplet merging.
While there’s no application for this novel discovery yet, the team is excited to see how this never-before-seen state can influence the field of soft-matter physics.
“Liquid-liquid mixtures are ubiquitous in consumer products and industrial processes,” says Paulsen. “This discovery, which offers a new approach to managing these mixtures, could one day help produce better products with longer shelf lives or save energy in chemical transport and processing. I’m eager to see the future implications of this breakthrough.”
This research was funded by the U.S. National Science Foundation and the U.S. Department of Energy.
Editor’s note: Portions of this article have been adapted from a UMass Amherst press release.
–Dan Bernardi
